Integrity, Excellence and Collaboration shape every aspect of our clinical trial support.
Zuellig Pharma aims to make healthcare more accessible and is dedicated to supporting the growing health care needs in Asia.A trusted leaders in clinical trial support services, We collaborate closely with our clients to deliver best-in-class clinical trial supply & logistics with comprehensive regional coverage. Our state-of-the-art facilities and globally compliant operations, combined with strong partnerships with industry stakeholders, ensure seamless access to essential healthcare resources. . 16+ Global depot network. 55+ Markets Coverage. Supports over 5,500 clinical trials globally. Clinical Trial Phase I through Phase IV, encompassing open-labelled, blinded, or randomised clinical trials.. Preferred clinical trial supply and logistics partner for Pharmaceutical, Biotech, CDMO, CRO and MNCs.
Zuellig Pharma
Taiwan's leading healthcare service company.
- The company boasts a state-of-the-art warehousing infrastructure in the Taoyuan Dayuan Area, which features the largest cold chain facility in Taiwan.
- This infrastructure is certified by ISO and complied with PIC/S to meet the guidelines for, Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP).
- Zuellig Pharma Taiwan has also helped the government distribute COVID-19 vaccines.
Established
1988
Medical-related customers
75
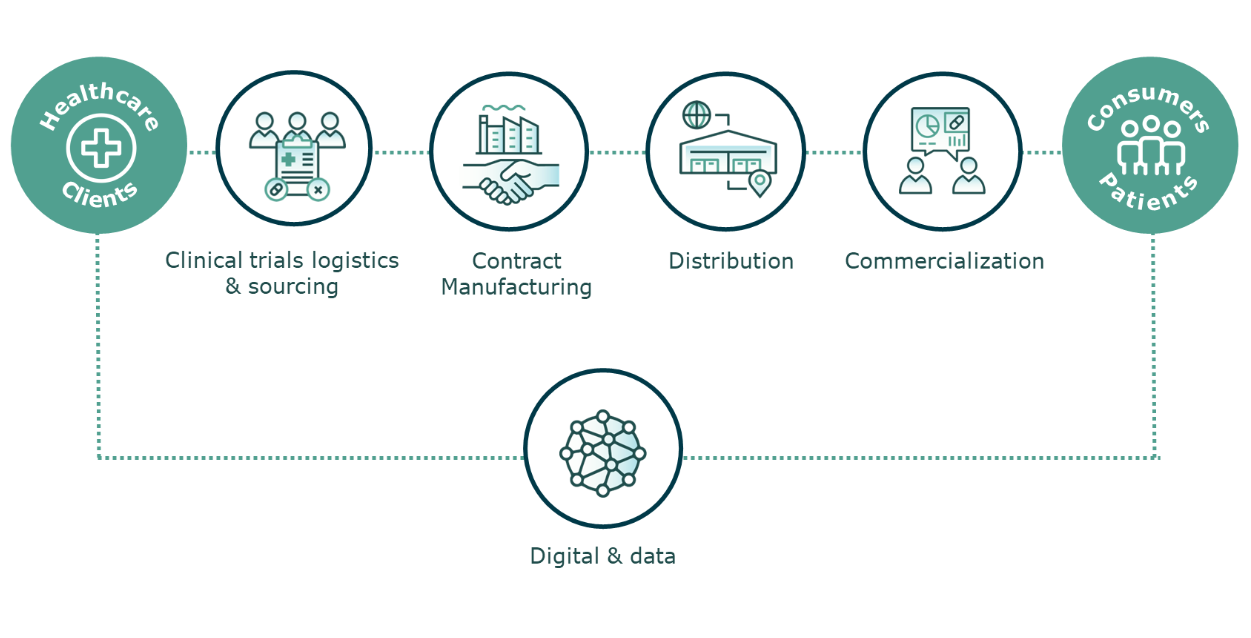
Leading Healthcare Solutions Company: Our Suite of Services.
We extend the clinical trial service to site management service in Taiwan.